Do you know you can make a pH indicator with the red leaves from a poinsettia plant? Red poinsettia leaves contain anthocyanins which change colour in the presence of acids or alkalis.
Acids have a low pH ( below 7 ), and alkalis have a high pH ( over 7 ).

If it’s not the season for red poinsettia leaves, red cabbage also makes a brilliant pH indicator.
You’ll need
A handful of poinsettia leaves
Pan
Water
Scissors
Sieve
Beaker
Test tubes or small transparent containers
Substances to test – lemon juice, vinegar, baking soda in water, water
Coffee filter – optional

Instructions
Place about 200ml of water in a pan and heat gently.
Cut a handful of red poinsettia leaves with the scissors and drop them into the pan.
Bring the water and poinsettia leaf mixture to the boil and then leave to stew for about 20 minutes.
Let the indicator cool and pour through the sieve into a beaker.
Pour a little indicator into two test tubes or small containers.
Add a couple of drops of your test substance and watch as the indicator changes colour.
The poinsettia indicator should turn pink/red in the presence of an acid and green if mixed with an alkali. I struggled to get my mixture to turn green but plan to try it again.
Poinsettia indicator strips
To make indicator strips with filter paper, soak a piece of filter paper or a coffee filter in the indicator mix and leave it to dry.
Cut the filter paper into strips and drop some of the test substances onto each strip. The strips should change colour.
What is a pH indicator?
Indicators are used to measure the pH of a substance. They change colour in the presence of an acid or alkali. The pH scale goes from 0 to 14.
Scientists commonly use Universal Indicator to measure pH. Universal indicator is a mixture of dyes that gives the colours below when an acid or alkali is added.
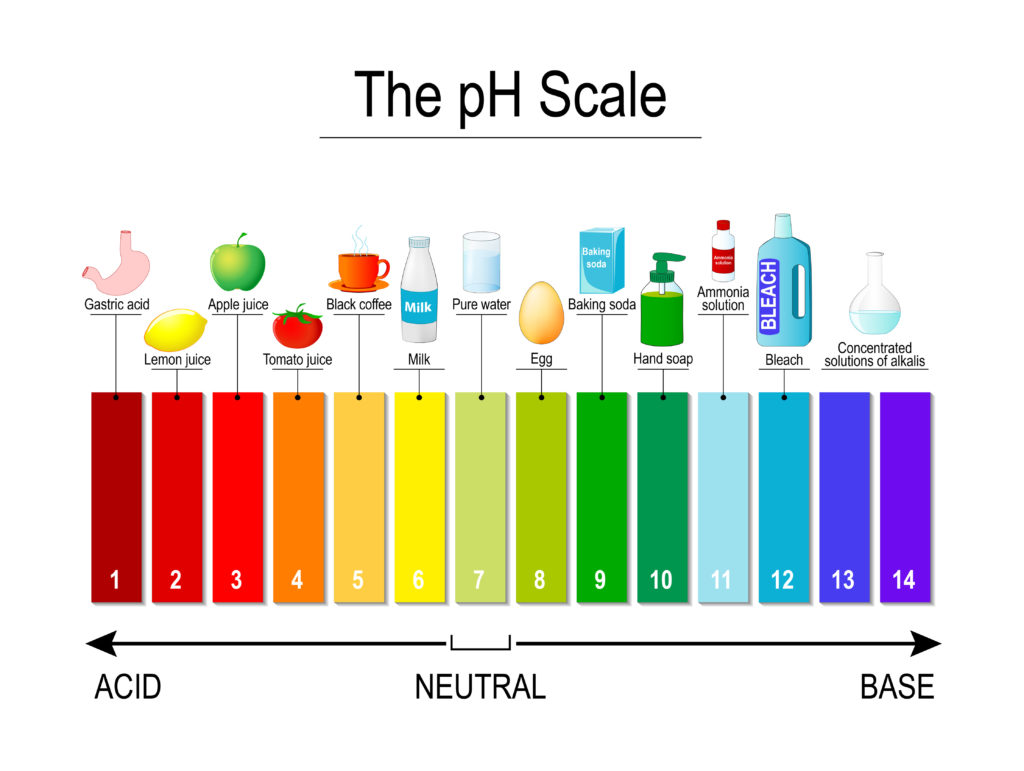
Examples of weak acids
Milk
Coffee
Tomato Juice
Examples of strong acids
Hydrochloric acid
Sulphuric acid ( found in car batteries )
Examples of weak alkalis
Washing up liquid
Examples of strong alkalis
Ammonia
Sodium hydroxide
All about acids and alkalis
Acids have a low pH ( below 7 )
Strong acids can be corrosive.
Acids are neutralised by bases ( alkalis ).
When acids react with some metals, they release hydrogen.
Alkalis have a pH above 7.
Alkalis can also be corrosive.
Alkalis neutralise acids to make salts and water.
An alkali is a soluble base!
Last Updated on December 29, 2022 by Emma Vanstone